A Real-Time Assay of Caspase 3/7 Activity in Cell Culture and Animal Models
Brock F. Binkowski and Braeden L. Butler
Promega Corporation
January 2014
Abstract
Methods
Circularly permuted firefly luciferase provides sensitive detection of protease activity, as demonstrated by the Protease-Glo™ Assay (Cat.# G9451). This cell-free approach offers a facile way of generating and screening numerous biosensor constructs containing peptide cleavage sites of variable length and composition, including proteases that require P´ residues (2). Although robust in a biochemical format, the biosensor construct associated with the Protease-Glo™ Assay showed reduced performance in cell-based assays.
We used directed evolution to develop a new firefly luciferase scaffold that shows superior performance for detection of protease activity in living cells. Like previous designs, a protease cleavage site is used to fuse the original N- and C-termini, and circular permutation is used to create new termini elsewhere in the structure (Figure 1). In the absence of cleavage, a steric constraint keeps the biosensor largely inactive. Following protease cleavage, the constraint is lifted, and large increases in luminescence result. A DEVDG cleavage site was used in this context to create a caspase-3/7 biosensor.

Results and Discussion
Monitoring Apoptosis in Cell Culture
Galbán et al. stably expressed the caspase-3/7 biosensor in 1833 breast cells and D54 glioma cells (1), which were then used to monitor caspase activation in tissue culture following addition of the cell-permeable substrate (GloSensor™ cAMP Reagent, Cat.# E1290).
Both cell lines showed large increases in luminescence following treatment with inducers of extrinsic and intrinsic apoptotic pathways (maximal induction of 20- to 200‑fold), with the kinetics of induction varying between insults. Biosensor activation was also used as a surrogate for apoptosis in a high-throughput screen for anticancer agents, demonstrating the utility of the assay to identify compounds that induce apoptosis with widely different kinetic profiles. Results from Promega also demonstrate the large differences in signal magnitude and kinetics that can be seen between different types of apoptotic insults (Figure 2, Panel A), results that were also seen following addition of Bright-Glo™ reagent (Cat.# E2650) to demonstrate use of an alternative assay format involving cell lysis (Figure 2, Panel B).

Monitoring Apoptosis in vivo
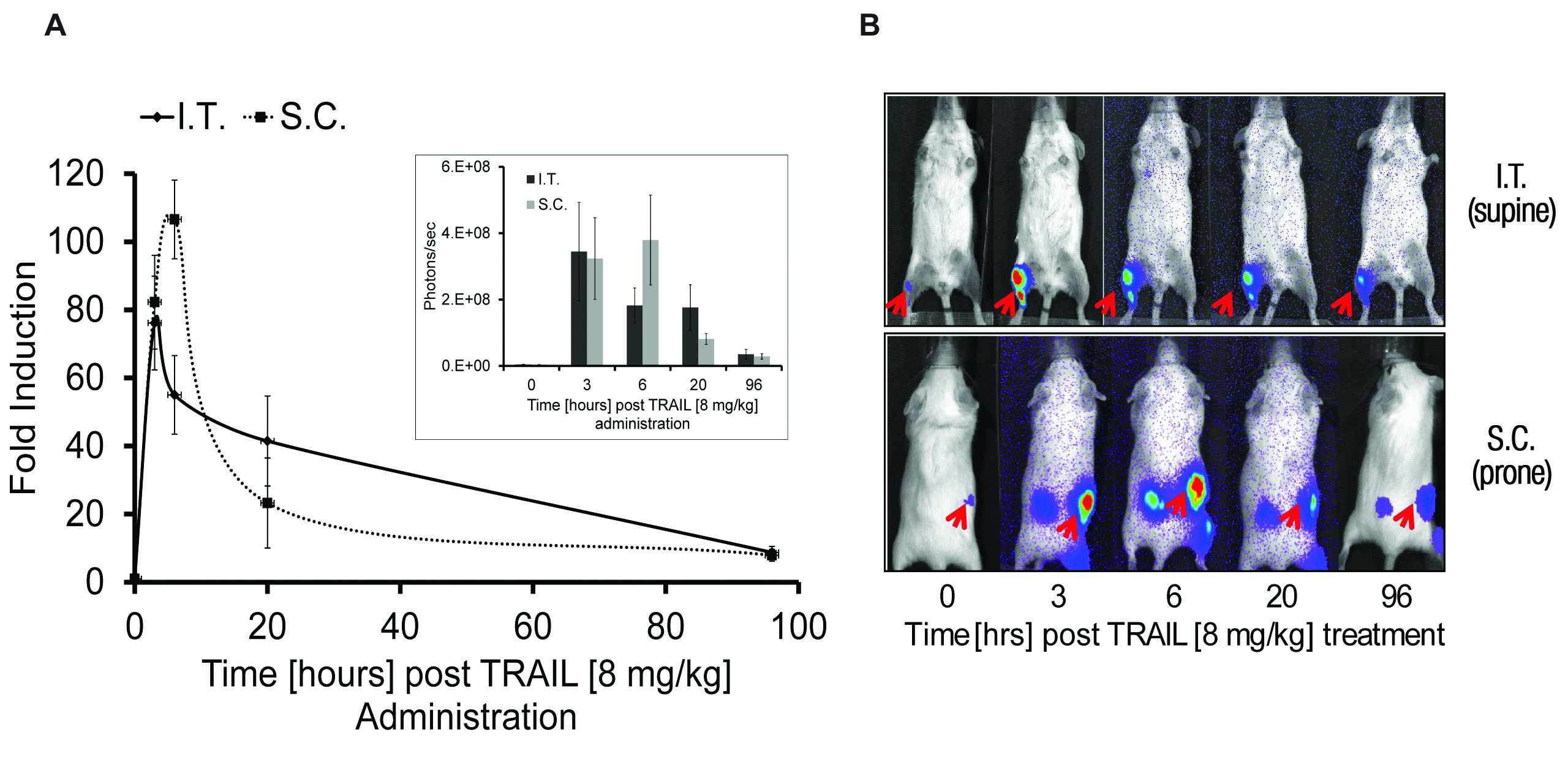
In SCID mice, Galbán et al. implanted xenografts of 1833 cells stably expressing caspase-3/7 biosensor and monitored caspase activation following treatment with inducers of apoptosis and interperitoneal injection of 150mg/kg of VivoGlo™ Luciferin (Cat.# P1041, Figure3). As observed with in vitro studies, large increases in luminescence were detected in vivo (maximal fold induction of 30- to 100-fold), and differences were seen in the caspase activation profile for inducers of extrinsic or intrinsic apoptotic pathways. To better facilitate work in vivo, Galbán et al. engineered a mouse to allow tissue specific expression of the caspase-3/7 biosensor via the CRE-Lox system, allowing sensitive detection of apoptosis in a tissue specific manner. Tissue or cell type specific expression of the biosensor in this context will allow the sensitive detection of apoptosis in a variety of in vivo model systems of human disease.
Conclusions
The caspase-3/7 biosensor described here provides sensitive and real-time detection of eff ector caspase activation in cultured cells. When used in vivo, it enables caspase activation to be monitored in select tissues or xenograft s with high sensitivity over a broad dynamic range, thus providing a potentially useful tool for therapeutic development. As with Protease-Glo™ Assay, the potential exists to extend the approach to a wide variety of protease targets both in vivo and in cell-based assays. For the latter, the approach was recently extended to viral proteases (3).
References
- Galbán, S. et al. (2013) Imaging proteolytic activity in live cells and animal models. PLOS ONE 8, e66248.
- Binkowski, B. et al. (2008) Novel genetically encoded biosensors using firefly luciferase. ACS Chem. Biol. 3, 346–51.
- Kilianski, A. et al. (2013) Assessing activity and inhibition of Middle East Respiratory Syndrome coronavirus papain-like and 3C-like proteases using luciferase-based biosensors. J. Virol. 87, 11955–62.
Learn more about the Caspase-Glo® 3/7 Assay System, which offers a convenient method to measure apoptosis in cell culture.