Lentivirus Tools
Lentiviruses are a type of retrovirus that can infect both dividing and non-dividing cells, making them an attractive vector for gene therapy applications. Using lentiviral vectors, therapeutic genes can be delivered into patient cells in order to stably replace, modify or supplement defective or missing genes from a patient's genome. Lentiviral vectors are also commonly used to deliver and express chimeric antigen receptor (CAR) in T cells and other immune cells.
Learn about our lentiviral vector tools, including a novel p24 immunoassay, in-process potency testing tools for T cell redirecting lentivirus and genomics solutions for lentivirus characterization and analysis.
We're here to help solve challenges in your research—just ask us!
Lentivirus Plasmid DNA Characterization
CAR-T cell therapy uses lentivirus vectors to modify T cells, allowing them to express a chimeric antigen receptor (CAR) that targets cancer cells. A critical step in this process is confirming the DNA sequence of the plasmid to ensure the correct genetic information is being introduced into the T cells. This step increases the therapy's effectiveness and ensures safety by minimizing the risk of unintended genetic alterations. See our recommended workflow for plasmid DNA sequencing below.
Download this flyer for details: Elevate Your CAR-T Therapy with Plasmid DNA Sanger Sequencing
Lentivirus Plasmid DNA Sequencing Workflow

Plasmid Purification

Amplification

PCR Cleanup

Quantitation

Cycle Sequencing

Sequencing Cleanup

Capillary Electrophoresis

DNA Analysis

Map of example sequence coverage. Four overlapping amplicons were prepared from an example CAR lentiviral transfer plasmid. The CAR construct sequencing was accurately completed with the ProDye® Terminator Sequencing System and Spectrum Compact CE System, achieving 4.15X coverage using fast sequencing protocol and 4.22X with standard sequencing protocol.
p24 Detection
p24 is a capsid protein of the lentivirus and can act as a surrogate marker for lentiviral particle count. p24 measurement is used to standardize and monitor vector production, thereby ensuring safety, quality, and consistency in the gene therapy development process.
Fast, Simple Measurement of p24
The Lumit® p24 Immunoassay is based on Lumit® technology, offering a simple, no-wash protocol, broad dynamic range and can be completed in as little as 30 minutes. It is a compelling alternative to conventional labor-intensive immunoassays such as ELISAs.
See protocol and data in this app note: Quantify p24 Protein in Lentivirus
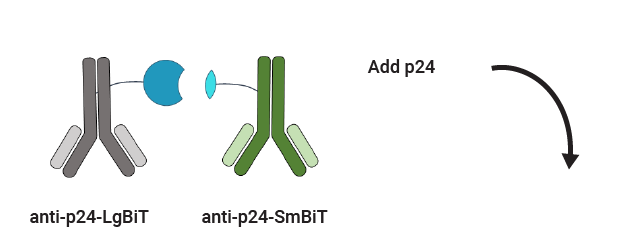
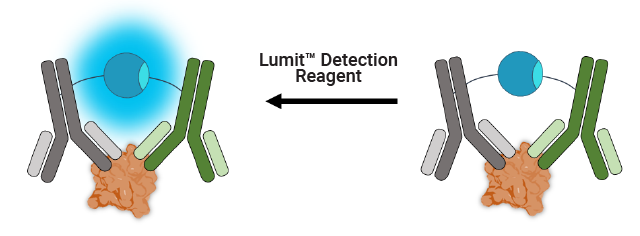
Lumit™ p24 Immunoassay Workflow

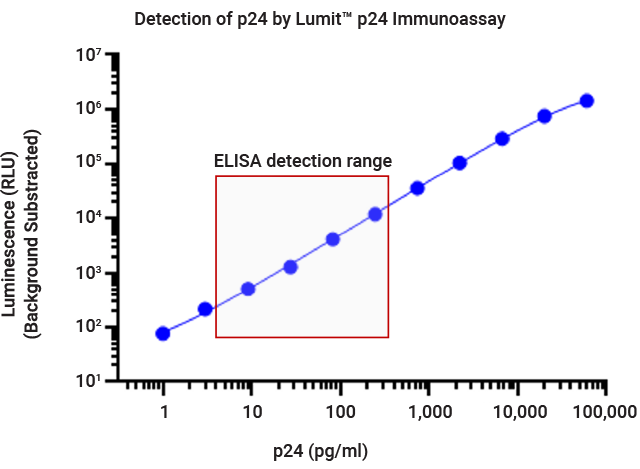

Measurement of p24 concentration using the Lumit® p24 Immunoassay. Twofold serial dilution of lentivirus samples were prepared in viral lysis buffer. X-axis represents the dilution factors of lentivirus from the stock samples. Dotted lines represent the upper and lower limits of the standard curve.
Lentivirus Genome Titer
Accurate quantification of viral titers allows researchers to standardize dosing, optimize transduction efficiency, and minimize the risk of adverse effects, such as insertional mutagenesis or immune responses.
Automated RNA or DNA Extraction for Lentiviral Vector Characterization
The Maxwell RSC® Instrument is magnetic particle mover that uses prefilled cartridges and pre-programmed methods to extract DNA and/or RNA from a wide variety of sample types in 25–60 minutes.
We also have high-throughput extraction chemistries and manual extraction solutions for RNA and DNA if you are working on a smaller scale.

Lentiviral Genome Titer Workflow

Lentivirus Dilution

RNA Extraction

RNA Quantification
Workflow of gDNA Extraction for Infectious Titer Determination After Cell Transduction
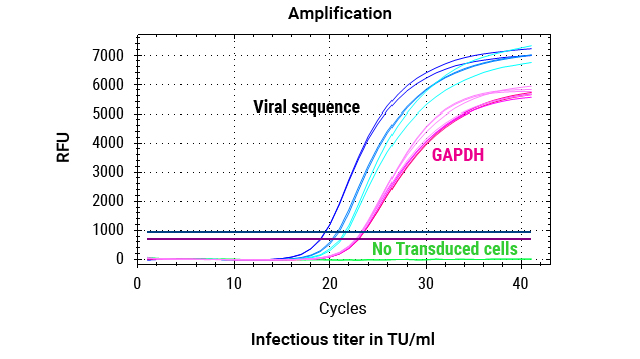
DNA was extracted from 2 million cells transduced with 3 dilutions (1:5, 1:10, 1:20) of lentiviral vectors, as well as from cells that were not transduced (no LV). The custom Promega One4All kit extracted high-quality gDNA with absorbance ratios higher than 2, and both the DNA quality and yields meet requirements for analysis.
Reverse Transcriptase Activity
Reverse transcription is a critical step in the lentiviral transduction process, where the viral RNA genome is converted into complementary DNA (cDNA) before integration into the host genome. Accurate measurement of reverse transcription activity is essential for optimizing vector production, ensuring transduction efficiency, and assessing vector potency and stability.
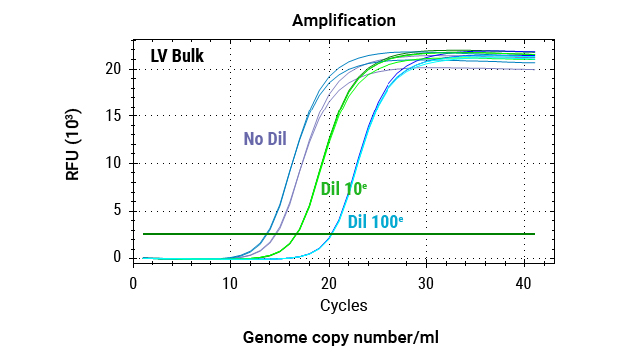
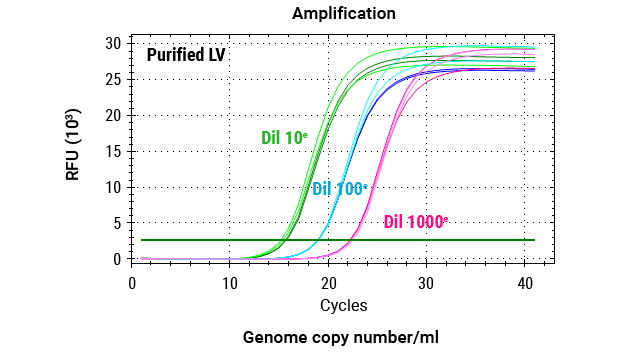
Quantification of Reverse Transcriptase Activity
Our XpressAmp® Direct Amplification Reagents and GoTaq® Probe RT-qPCR Master Mix can be used in a product-enhanced reverse transcriptase (PERT) assay format to sensitively and quantitatively measure lentivirus reverse transcriptase activity.

Quantitative PCR analysis of MS2 RNA amplification across a serial dilution of lentivirus. Reactions were performed using 80ng MS2 RNA, 0.5X XpressAmp® Lysis Buffer with 1-thioglycerol, and GoTaq® Probe RT-qPCR Master Mix. Triplicate amplifications were conducted for each dilution point.
Potency Testing
For lentivirus vectors used in CAR-T manufacturing, regulatory authorities recommend additional measures of potency beyond transgene expression of the viral vector in CAR-T cells. This ensures that the viral vector retains its functional ability to transduce cells and express the CAR.
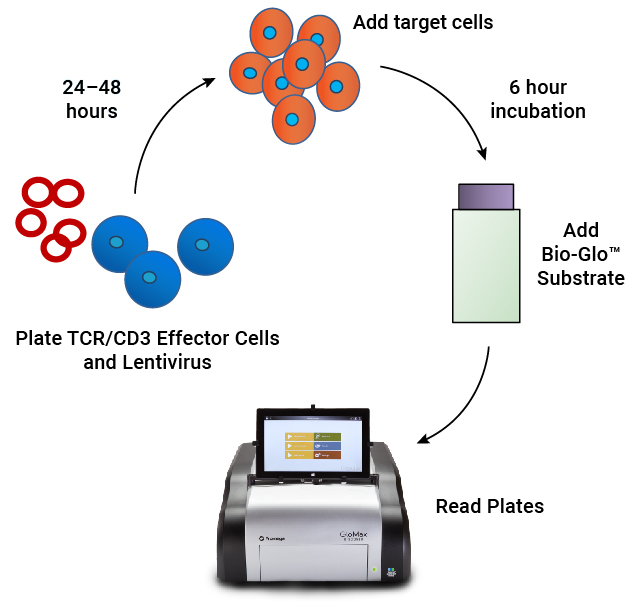
In-Process Potency Testing of T Cell Redirecting Lenti-CAR Virus
Our T Cell Activation Bioassay is an MoA-based potency assay used to measure CAR:target cell engagement for T cell redirecting lentiviral vectors. It provides a quantitative, stability-indicating luminescent readout that can be implemented as part of your CAR-T workflow.
See more data in this publication: A bioluminescent reporter bioassay for in-process assessment of chimeric antigen receptor lentiviral vector potency.
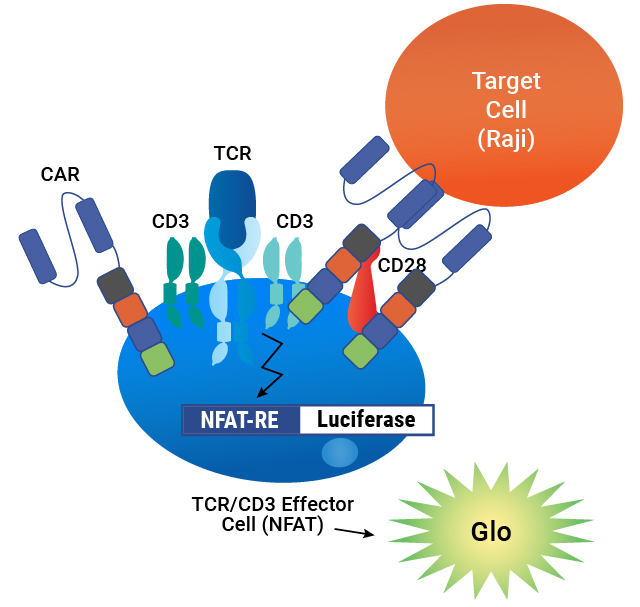
Lentivirus Bioassay is Specific


Lentivirus Bioassay is Stability Indicating

| Heat Treatment Time (hours) |
EC50 |
% Inhibition of Max |
|---|---|---|
| 0 |
0.81 | Reference |
| 2 |
1.0 |
26% |
| 6 |
2.4 |
18% |
| 24 | 2.5 |
47% |
| 48 | 5.0 |
67% |
Forced degradation samples of CAR-19 lentivirus were prepared by incubating at 37°C for 2–48 hours, then analyzed using TCR/CD3 Effector Cells and Raji Target Cells. The assay demonstrates a loss of lentiviral potency with heat treatment, as measured by an increase in EC50 and a decrease in the maximum response.